Understanding Pain
Rey Allen & Marie Zahn, Certified Rolfers
During the past twenty years, research on chronic pain has significantly increased, with considerable advances in understanding its etiology, assessment, and treatment. These discoveries have important healthcare implications as pain is one of the leading causes for people to seek out medical care. Whether or not pain relief is your immediate goal, the fact remains that the majority of people who walk into your office experience some degree of pain and/or tension. As such, we must understand what pain is, and more importantly, what pain is not. For manual and movement therapists, this is no less important than knowing the anatomy of the body. This article is a review of the most recent scientific understanding of pain. This information is both relevant and applicable to you and your clients, as it proposes explanations for why, with treatment, a client’s pain may decrease, remain the same, or gets worse. Understanding these phenomena is empowering to both you and your clients.
Within roughly the last twenty years, neuroscience and pain science have discredited the belief that pain reflects the state of physical tissues (i.e. pain = tissue damage), a purely biomechanical explanation for pain (Gifford 1998, Lederman 2010). The International Association for the Study of Pain (IASP) defines pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.” Simply put, pain is the brain’s perception of tissue damage (Butler and Moseley 2003). Perception is key, because pain is about how a person consciously and non-consciously creates meaning of his/her physical reality. This perception of tissue damage (i.e. pain) is modulated by a number of cognitive, emotional, and sensory inputs (Gifford 1998; Carlino et al. 2014).
When someone in pain walks into your office, they want answers to the following:
1. What’s wrong with me?
2. How long is this going to last?
3. Is there anything I (the client) can do about it?
4. Is there anything you (the practitioner) can do about it?
(Verbeek et al. 2004; Gifford 2014)
These questions are prompted by the underlying assumption that pain is the indicator for something “wrong” in the physical body. A number of hypotheses will be made (joint misalignment, degeneration, compression) so that a treatment regime is identified to “fix” these physical morbidities. When experiencing pain we sensibly, but mistakenly, place all of our attention in the physical domain. Today, we see the falsification of the conclusion that pain predictably represents tissue damage.
“Pain is an opinion on the organism’s state of health rather than a mere reflexive response to injury. The brain gathers evidence from many sources before triggering pain.” -V.S. Ramachandran
PERCEPTION VS. STATE OF TISSUES
One of the brain’s chief priorities is to keep us safe and protected. Pain warns us of danger and compels us to take action to relieve and/or avoid that danger. Thus, the experience of pain is based on a prediction of danger that we are physically in, not how much we are actually in. Even if there are no problems in the tissues, nerves, or immune system, you can still hurt if your brain concludes that you are in danger (Butler & Moseley 2003).
Historically, a class of sensory receptors called nociceptors were once, incorrectly, referred to as pain receptors. Nociceptors are receptors that require higher thresholds of stimuli to trigger an action potential, which in turn sends larger, more amplified signals to the central nervous system (CNS). These larger signals serve to get a person’s attention by acting as warning signals. The brain, though, can ignore input from the body, large or small, if the brain is either distracted enough or does not value the incoming messages. Pain is context-dependent. A notable example of this is seen with soldiers in the heat of battle who are shot but don’t feel pain until much later, once they are out of the dangerous environment. Nociception is quantitative, not qualitative. This means the body’s sensory system simply provides raw data to an individual's CNS. It is the brain that then interprets the data’s meanings through predictive coding, resulting in a physical sensation (a perception) which may or may not include pain. While nociception is not an essential part of one's pain experience, it unmistakably can be a very powerful contributor.
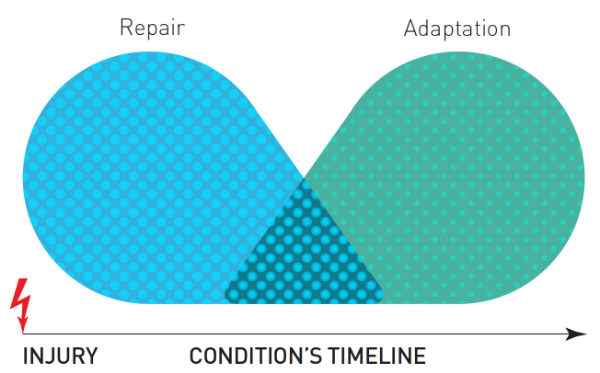
If a person's nervous system has not attenuated after the determinant time for healing of the injured tissue, the brain continues to conclude a state of threat and what formerly was acute pain is now chronic (Lederman 2015). The healing of damaged tissue is a complex and dynamic process, consisting of four primary phases: blood clotting (hemostasis), inflammation, tissue grown (proliferation), and tissue remodeling (maturation) Diegelmann and Evans 2004; Watson 2016). Typically, most tissues heal within 1-6 months. Soft tissues, such as skin, takes around 10 days to 2 weeks. Deeper soft tissue can take 3-6 months, and depending on the type of deeper tissue, it may take up to a year to regain full tensile strength. Bone takes up to 3-6 months to heal and up to a year to fully remodel. Recovery from tissue damage includes the resolution of healing (particularly inflammation) and attenuation of nociception excitation. The point is, though, that a time frame exists within which all tissues complete their healing phases. Conditions that arrest normal would healing are rare but include malnuturiction, metabolic disease, circulation problems, infection or an irregular reaction. In some cases, certain medications interfere with tissue and would healing. Pain serves an important role during healing to ensure the process is preserved, and to prevent further tissue damage in order to facilitate a full recovery (Lederman 2015; Figure 1). If pain persist past the healing window, it is considered chronic. It is important to reiterate, though, that an individual can experience an acute pain when no tissue damage is present. We can refer to this more accurately as a "pain event."
Figure 1: Overlap from repair to adaptation. Adapted from Fig. 3 in Lederman (2015).
Unfortunately, many healthcare providers do not clearly communicate or adequately educate their clients that all tissues eventually heal and that the severity of pain and the state of tissue damage do not positively correlated (Moseley 2003; Louw et al. 2012). Many clients, and some clinicians, wrongly believe that once a part of their body is are injured, that part will always be in a state of injury. In other words, experiencing episodic pain is considered to be an episodic injury, as seen with the term “re-injury." What instead is likely to be happening is simply the reoccurrence of neuronal excitation for that region of the body. In other words, the region is not being damaged again.
Changes are not only happening in the nervous system itself, but people move differently when experiencing pain. Of course this statement is obvious when considering our personal accounts of modifying behavior in response to pain. Clients may think pain and muscular tension are directly coupled, where one may cause further intensification of the other in a vicious cycle. Hodges and Tucker (2011) refute the idea that there is a predictable, uniform increase in of muscle activity with the presence of pain; instead, they propose pain has variable effects on an individual’s motor output. With the overall goal of protecting the painful part from further injury or danger, a redistribution of motor activity occurs that can be additive, competitive, or complementary within numerous sites of a motor pathway (Hodges and Tucker 2011). In other words, a number of muscular adaptations may occur in response to pain and are also dependent on the individual and the given physical task; some include: increase or decrease in muscle activity, poor proprioception, or altered coordination. They explain how physical adaptations to pain, like modified movement or stiffness, have short-term benefits but long-term consequences—that may or may not be an issue in the future—due to the changes in load distribution (Hodges and Tucker 2011).
A difficult aspect in understanding pain is that how it is not a sequential or linear process, but rather an emergent process of neuronal activity, originating from a variety of inputs. Pain is a substantial sensory and emotional experience that is modulated by psychological, social, and contextual factors—now defined by the “biopsychosocial” model (Carlino et al. 2014).
THE BIOPSYCHOSOCIAL MODEL
Pain should never be seen as context-free but rather context-dependent (Turk & Monarch 2002; Carrio et al. 2004; Gatchel 2004). Biopsychosocial research clearly shows that pain is a complex experience that never has a single stimulus (Figure 2). Pain may involve sensory, motor, autonomic, endocrine, immune, cognitive, affective, and behavioral components (Gatchel et al. 2007). Pain is filtered through an individual’s genetic composition, prior learning history, current psychological status, and socio-cultural influences. Thus, tissue damage can occur in the absence of pain. It helps to see the CNS/brain as the central scrutinizing center, continuously sampling (conscious and non-conscious) from its environment, body, and past experiences (Gifford 1998; Figure 2).
The biopsychosocial model (BPS) for chronic pain is a non-dualistic and integrated approach (Engel 1981). BPS is based on a systems approach as well as a way of understanding the client’s subjective experience as an essential contributor to diagnostic accuracy, health outcomes, and human care (Gifford 1998; Carrió 2004). The biological system (bio-) deals with anatomical and molecular substrates of disease—the client’s physiology. The psychological system (psycho-) includes the effects of psychodynamic factors like motivation, attitudes, and personality on the experience of and reaction to illness and pain. The social system (-social) examines the cultural, environmental, economic, familial, and social circumstances surrounding the expression and experience of illness and pain (Dogar 2007).
Figure 3: Mature Organism Model. Adapted from Fig. 2 in Gifford (1998).
STRESS AND ANXIETY
While stress and anxiety commonly overlap, individually they aren’t the same. Stress is a state of mental, emotional, and/or physical arousal due to demanding and adverse circumstances from the environment. This state of arousal results in an intense focus of attention, which may also serve as a distraction from pain. Acute Stress (short term) activates the endogenous opioid system which raises the pain threshold, sometimes called “stress-induced analgesia,” meaning less sensitivity Conversely, anxiety is defined by worry, apprehension, and anticipation of impending events or future pain. Anxiety lowers pain thresholds and results in hyperalgesia, sometimes called “anxiety-induced hyperalgesia,” which means heightened sensitivity (Carlino et al. 2014).
For many, the difference between acute and chronic states of stress only includes the temporal dimension, but there is an important physiological distinction between the two. Short-term (acute) stress scenarios trigger the secretion of cortisol from the adrenal cortex to increase metabolism and blood sugar levels. This provides the energy necessary for the body to mobilize, responding to a threat and restoring homeostatic balance (“fight or flight” response; Gatchel et al. 2007). However, in states of chronic stress, and thus prolonged periods of homeostatic imbalance, deleterious physiological consequences develop, including muscular atrophy, impairment of tissue growth and repair, suppression of the immune system, and morphological changes in brain structures. These conditions may be the foundation for developing and maintaining chronic pain states.
Those suffering with chronic pain often have other forms of emotional and physical symptoms: anxiety, stress, depression, loss of appetite, fatigue, insomnia, tension, and fear avoidance (Lunderg 2011). While pain can incite emotions, emotions can conjure pain (Cervero 2012). It is normal to be concerned or even anxious about the cause of our pain and its potential for future disability. However, anxiety has a great influence on the maintenance, if not exacerbation, of chronic pain (Eccleston and Crombez 2007).
“Following injury, or any noxious event, people’s feelings or emotions and thoughts about their situation will change. Injury and the consequent of pain may produce powerful aversive feelings of fear, anxiety or increasing anger.” -Louis Gifford
THE NEUROMATRIX THEORY OF PAIN
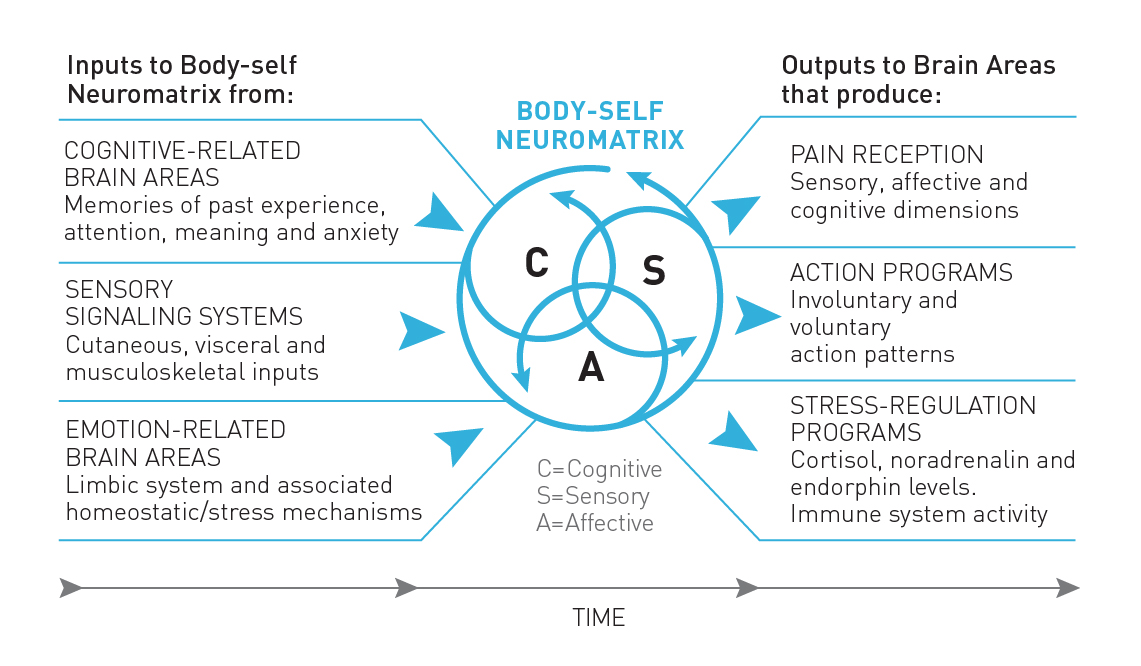
The current and widely accepted theory of pain is the neuromatrix theory of pain, developed by Ronald Melzack (2001; Figure 3). The body-self neuromatrix is defined as an anatomical substrate for the widespread neuronal network processes that are sculpted by a multitude of sensory inputs. It reflects the emergent processes of neuronal activity in the brain. When there are repeated cyclical processes and syntheses of nerve impulses through the neuromatrix, the neuromatrix imparts a particular characteristic pattern emerges, called a neurosignature. In other words, a neurosignature is a particular specific neuronal pattern in within the neuromatrix.
Figure 4: The Neuromatrix Theory of Pain. Adapted from Fig. 1 in Melzack (2001).
The neuromatrix theory of pain proposes that pain is an imprint, or “pain neurosignature,” of nerve impulse patterns that are generated by the body-self neuromatrix (Melzack 2001). There are many inputs to the brain that can create or later trigger a pain neurosignature, including movements, thoughts, emotions, touch, memories, fears, smells, and visual stimuli, to name a few. Interestingly, the neuromatrix requires no actual sensory input for a person to experience pain, only the activation of a pain neurosignature; phantom limb pain is an example of this.
The most important takeaway from the neuromatrix theory of pain is recognizing that pain is an output of the brain rather than being dependent on or a response to sensory input like damaged tissue (Melzack 2001; Gatchel et al. 2007).
EVIDENCE
Always remember: correlation does not imply causation. A recent systematic review of 33 papers with a total of 3,110 subjects published in the American Society of Neuroradiology (Brinjikji et al. 2015) found absence of pain associated with spine degeneration. The occurrence of disc degeneration in healthy, pain-free individuals ranged from 37% of subjects in their 20s to 96% of those 80 years of age (Table 1). Similarly, 30% of those in their 20s had bulging discs. MRI findings of degenerative changes in the spine and discs are part of the normal aging process rather than a disease or the cause of pain. The results from Brinjikji et al. (2015) showing 50% of asymptomatic individuals 30-39 years of age have disc degeneration, height loss, or disc bulging suggest that even in young adults, degenerative changes may be incidental and not causally related to presenting symptoms. This study strongly suggests that when degenerative spine findings are observed, it may be normal age-related changes rather than a pathology.
Table 1: Spine images of asymptomatic people. Adapted from Table 2 in Brinjikji et al. (2015)
A study published in Spine (Nakashima et al. 2015) observed MRI findings of cervical spines of 1,211 healthy, asymptomatic Japanese adults between the ages of 20-70. Surprisingly, 73.3% and 78.0% of males and females, respectively, in their 20s had bulging discs, though only 5.3% of all of these asymptomatic subjects were diagnosed with spinal cord compression and increased signal intensity. The conclusion of Nakashima et al. (2015) was that it is dangerous to make interventional decisions based on findings in MRI images alone. The lesson: It is a mistake for our treatment decisions to be based on image findings alone. Hurt does not necessarily equal harm.
Finally, Del Grande et al. (2016) studied professional baseball pitchers without pain using high-resolution 3-T MRI and found substantial soft tissue abnormalities. Out of the 19 asymptomatic (no pain) individuals studied, 68% presented tendinopathy, 21% acromioclavicular joint osteoarthritis, and 32% showed partial thickness supraspinatus tendon tears. For professional athletes, MRI images are used widely to provide evidence for pathologies in order to inform the cause of their symptoms (typically pain or a decrease in performance) and effective treatment. Del Grande et al. (2016) provides further confirmation of how tissue abnormalities can exist—in this case, specifically 19 baseball pitchers—without the presence of pain. Consequently, the important takeaway is that lesions found in MRI reports can be misinterpreted for symptomatic pathologies.
“Think about it: A patient comes to you seeking help for pain, and you teach the patient anatomy! No wonder pain rates in the U.S. have doubled in the last 15 years alone. Never before have we performed as much surgery or prescribed as much medicine for pain in the history of mankind, and pain rates are ever increasing.” -Adriaan Louw, PT, PhD
KNOWLEDGE IS POWER
When a client understands how pain works, they can bring conscious awareness to the triggers of their pain, and in doing so, they can better manage and likely decrease their pain. Changes in opinion provides clients with more adaptive thoughts and behavior. Research conducted by Louw et al. (2014) showed that giving people a pain education lessens their pain experience post-surgery and reduces medical expenses by 45%. Additionally, Louw et al. (2012) showed that for patients, knowing how a surgical procedure will affect their symptoms is for them the most important. Some practitioners think that their clients cannot understand pain physiology. However, Moseley (2003) showed health professionals inaccurately estimate client’s ability to understand the neurophysiology of pain and that despite clinicians’ beliefs that patients aren’t able to understand pain physiology, clients can and want to understand their pain.
Understanding pain gives clients confidence and self-efficacy in knowing that their bodies are sensitive, not vulnerable or fragile. Self-efficacy for people with chronic pain is when they have the internal resources to carry out certain activities or achieve a desired outcome in spite of experiencing pain (Lee et al. 2015). In other words, for a person seeking to engage in a particular behavior or activity, they’re more likely to do that if they feel like they have the internal resources to perform, regardless of their pain.
With 172 subjects, Costa et al. (2010) examined whether pain self-efficacy and/or fear of movement mediated the relationship between pain intensity and the disability of pain. What they found was that beliefs of pain self-efficacy and fear of movement partially mediated the effects of pain intensity and disability at the onset of pain. Moreover, 12 months after the onset of low back pain, subjects with high self-efficacy possessed less pain intensity and disability. Additional studies support the relationship between higher patient self-efficacy and lower levels of pain and disability (Reid et. al 2003; Denison et al. 2004; Dohnke et al. 2005). These conclusions were supported in a systematic review and meta-analysis examined by Lee et al. (2015); this study involved 2961 subjects where the research team tested what roles of fear, catastrophization, self-efficacy, and other variables such as psychological distress (depression and anxiety) have in determining disability with people with low back pain and neck pain. While all of these variables interrelate, they found that self-efficacy was the strongest mediator, followed by psychological distress, and then fear of pain.
Practice makes perfect. Rehearsing any task results in higher proficiency of that task. This is a rule of neuroplasticity. The same applies if our attention is always focused on our pain: the nervous system, at all levels, will alter itself physically and chemically to be more sensitive, also known as long-term potentiation. As pain persists, the nervous system becomes better at producing pain. What was previously considered to be an innocuous stimulus may now be perceived as noxious, making it harder for ordinary central nervous system (CNS) inhibitory mechanisms to operate effectively (Kwon et al. 2014).
Teaching a person with chronic pain the science of their pain is a cognitive behavioral management tool and treatment target. If done well, it has the potential to reduce clients' fears, which likely decreases pain intensity and lowers overall disability. Although a pain education may not necessarily reduce or eliminate pain, it supports the intended goals in all forms of therapies in motivating clients to play a more active role in their recovery (Nicholas 1989; Altmaier et al. 1993). That said, pain education is no easy task. It must be appreciated how deep-seated human beliefs are and the preconceived ideas we have of our bodies. Through a willingness to commit time and effort to properly educate clients, clinicians can have a profound effect on empowering clients to get back to living life more fully (Lederman 2015).
“Don’t move the way fear makes you move.” –Rumi
CONCLUSIONS
Our experience of pain is a top-down process—always. In fact, there is no such thing as myofascial pain, bone pain, organ pain, or even the existence of nerve pain. There’s just pain. This means damaged and pinched nerves do not have to hurt. Even in the presence of actual tissue damage—given pain is an output of the brain—it is our brain that concludes whether our tissues are in danger.
Pain is primarily a psychological experience (Craig and Hadjistavropoulos 2004). This is not to say that pain is all in your head, as in your imagination, but it is a construct of the brain projected onto the body. Modern pain science does NOT imply people imagine their pain. Pain is real. Pain is always real. Pain literally changes our PNS and CNS physically and chemically. This is the dark side of neuroplasticity.
The terms “acute” and “chronic” are temporal identifiers, not characteristic of whether tissue damage is present or not. In other words, someone can have acute pain without any tissue injury, and so their recovery from that acute pain is not comprised of tissue repair, but instead is the attenuation of the pain itself. One of the most important factors to demonstrate the difference between acute and chronic pain is injury healing times and their relevancy. The intensity of acute pain is commonly interpreted to reflect the severity of tissue damage; in other words, any quick onset of pain is considered acute, with or without tissue injury. However, in both acute and chronic conditions, pain is still the brain's interpretation of physical danger.
Pain science not only reveals our misunderstanding about how pain works but also examines how our common vernacular as practitioners can cause more hurt in the end. First, we must be careful not to discount our client's pain experience, even if we think we already have an understanding of their false beliefs. Commonly, clinicians feel rushed to get the educational portion of their treatment over with but this can only serve to further invalidate a client's pain experience. By not allotting time to fully listen to our client's complaints, stories, and values, we run the risk of invalidating their pain and truly misunderstand who they are as a victim of pain (Wernicke et al. 2015).
Secondly, we should be mindful of our speech. Are we using terms that incite fear and worry? Additionally, if we refer to pain as an experience (verb) rather than a thing (noun), we signal to our clients that their pain is a state that has the ability to change. This encourages a more active enrollment from our clients in their recovery. This also provides insight for them to see that their thoughts and behaviors lead to positive or negative consequences. Sadly, referring to pain as a noun misplaces the multi-factorial experience that pain is. Pain as a noun also can mislead both practitioners and clients to think pain is in tissues thus can be removed from their tissues (noun). If we're speaking in wrong terms, we're thinking in wrong terms.
Whether experiencing chronic or acute pain, incessant worry, fear avoidance, and self-diagnosing only further sensitizes one’s nervous system. When false beliefs are determined as the root of causing harm, providing client education is one of the most effective interventions. At the same time, we don’t want to cause too much cognitive dissonance at first when explaining pain. We must take special care when educating clients who seem to use their pain to self-identify.
Above all, build self-efficacy and explain pain on purpose!
“To reduce pain, we need to reduce credible evidence of danger and increase credible evidence of safety.” –Dr. Lorimer Mosely
HOW TO GET STARTED:
• First, listen to your clients and their stories, ensuring that they know you care. The single most important factor linked to a client’s improvement is not the thoroughness of the medical history or physical assessment. Instead, it is whether or not clients indicated that the practitioner had carefully listened to their descriptions of their condition on the first visit.
• Provide reassurance that they and their bodies’ tissues are okay, because tissues are adaptable and resilient. Remember, all tissues complete their healing phase.
• Correct any false beliefs about pain that you hear coming from the client throughout treatment, as this is a paradigm shift for most people. Know that not everyone will get it at first, so be kind, patient, and persistent.
• Encourage clients to police their thoughts of worry and to strengthen their social network, seeking out fun and laughter.
• Hurt doesn’t necessarily equal harm. It is worth reminding our clients that an entirely pain-free life is an unreasonable expectation, so keep moving!
• Strongly advise that they have an exercise regime that matches their tolerances, grading their exposure to incrementally increase load over time. Exercise serves as an analgesia, building clients' confidence in their tissues (Gurevich et al. 1994, Naugle et al. 2012, Nijs et al. 2015). You can refer to a physio or personal trainer who is up to date with pain science so that the message is confirmed and consistent.
• Build self-efficacy!
REFERENCES
Altmaier, E. M., D. W. Russel, C. F. Kao, T. R. Lehmann, J. N. Weinstein (1993). Role of self-efficacy in rehabilitation outcome among chronic low back pain patients. Journal of Counsel Psychology, 40: 335-339.
Brinjikji, W., P. H. Luetmer, B. Comstock, et al. (2015). Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. AJNR. American Journal of Neuroradiology, 36(4): 811–816.
Butler, D. and L. Moseley (2003). Explain Pain. Noigroup, Adelaide, Australia. Print.
Carlino, E., E. Frisaldi, F. Benedetti (2014). Pain and the context. Nat. Rev. Rheumatol, 10: 348–355.
Cervero, F. (2012). Understanding Pain. Massachusetts: The MIT Press.
Carrio, F. B., A. Suchman, R. Epstein (2004). The biopsychosocial model 25 years later: principles, practice, and scientific inquiry. Annals of Family Medicine, 2(6): 576–582.
Costa, L., C. Maher, J. McAuley, et al. (2010). Self-efficacy is more important than fear of movement in mediating the relationship between pain and disability in chronic low back pain. European Journal of Pain, 15(2): 213-219.
Craig, K., T. Hadjistavropoulos (2004). Pain: psychological perspectives. Mahwah, NJ: Lawrence Erlbaum Assoc. Print.
Del Grande, F., M. Aro, S. J. Farahani, A. Cosgarea, J. Wilckens, J. A. Carrino (2016). High-Resolution 3-T Magnetic Resonance Imaging of the Shoulder in Nonsymptomatic Professional Baseball Pitcher Draft Picks. J Comput Assist Tomogr 40(1): 118-125.
Denison, E., P. Asenlof, P. Lindberg (2004). Self-efficacy, fear avoidance, and pain intensity as predictors of disability in subacute and chronic musculoskeletal pain patients in primary health care. Pain, 111:245-252.
Diegelmann, R. F., M. C. Evans (2004). Wound healing: an overview of acute, fibrotic and delayed healing. Frontiers in Bioscience, 9:283-289.
Dogar, I. A. (2007). Biopsychosocial model. Annals of Punjab Medical College, 1(1): 11-13.
Dohnke, B., B. Knäuper, W. Müller-Fahrnow (2005). Perceived self-efficacy gained from, and health effects of, a rehabilitation program after hip joint replacement. Arthritis Care Res, 53: 585-592.
Eccleston, C., G. Crombez (2007). Worry and chronic pain: a misdirected problem solving model. Pain, 132: 233–236.
Engel, G. L. (1981). The clinical application of the biopsychosocial model. The Journal of Medicine and Philosology, 6 (2): 101-124.
Gatchel, R. J. (2004). Comorbidity of chronic pain and mental health: The biopsychosocial perspective. American Psychologist, 59: 792–794.
Gatchel, R. J., Y. Peng, M. Peters, P. Fuchs, D. Turk. (2007). The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychological bulletin, 133.4: 581-624.
Gifford, L. (1998). Pain, the tissues and the nervous system: a conceptual model. Physiotherapy, 84 (1).
Gifford, L. (2014). Aches and Pains. Book 1 of 3, Section 10. Dalmouth, Cornwall, CNS Press. Print.
Gurevich, M., P. M. Kohn, C. Davis (1994). Exercise-induced analgesia and the role of reactivity in pain sensitivity. J Sports Sci., 12(6):549-59.
Hodges, P. W., and K. Tucker (2011). Moving differently in pain: a new theory to explain the adaptation to pain. Pain 152:S90-S98.
Kwon M., M. Altin, H. Duenas, L. Alev (2014). The role of descending inhibitory pathways on chronic pain modulation and clinical implications. Pain Practice, 14 (7) :656-67.
Lederman, E. (2010). The fall of the postural-structural-biomechancial model in manual and physical therapies: exemplified by lower back pain. CPDO Online Journal, 1-14.
Lederman, E. (2015). A process approach in manual and physical therapies: beyond the structural model. CPDO Online Journal, 1-18.
Lee, H., M. Hubscher, G. L. Moseley, et al. (2015). How does pain lead to disability? A systematic review and meta-analysis of mediation studies in people with back and neck pain. Pain journal, 156: 988–997.
Louw, A., D. Butler, I. Diener, E. Puentedura (2012). Preoperative education for lumbar radiculopathy: A survey of US spine surgeons. Int J Spine Surg., 1 (6): 130-9.
Louw, A., E. Puentedura (2013). Therapeutic neruoscience education, teaching patients about pain. USA: International Spine and Pain Institute. Print.
Louw, A., I. Diener, M. R. Landers, E. J. Puentedura (2014). Preoperative pain neuroscience education for lumbar radiculopathy: a multicenter randomized controlled trial with 1-year follow-up. Spine, 39(18):1449-57.
Lunderg, M., A. Grimby-Ekman, J. Verbunt, M. J. Simmonds (2011). Pain-related fear: a critical review of the related measures. Pain Research and Treatment.
Maxson, S., E. Lopez, D. Yoo, M. A. Leroux, et al. (2012) Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl. Med., 1(2):142-9.
Melzack, R. (2001). Pain and the neuromatrix in the brain. Journal of Dental Education, 65(12): 1378-1382.
Moseley, L. (2003). Unraveling the barriers to reconceptualization of the problem in chronic pain: The actual and perceived ability of patients and health professionals to understand the neurophysiology. Journal of Pain 4(4):184-189.
Nakashima, H., Y. Yukawa, K. Suda, M. Yamagata, T. Ueta, F. Kato. (2015). Abnormal findings on magnetic resonance images of the cervical spines in 1211 asymptomatic subjects. Spine, 40(6):392-8.
Naugle, K., R. Fillingim, J. Riley (2012). A meta-analytic review of the hypoalgesic effects of exercise. The Journal of Pain, 13(12): 1139-1150.
Nicholas, M. K. (1989). Self-efficacy and chronic pain. Paper presentation at the annual conference of the British psychological society, St. Andrews, Scotland.
Nijs, J., E. Lluch Girb, M. Lundberg, A. Malfliet, M. Sterling (2015). Exercise therapy for chronic musculoskeletal pain: Innovation by altering pain memories. Manual Therapy, 20: 216-220.
Reid, M. C., C. S. Williams, T. M. Gill (2003). The relationship between psychological factors and disabling musculoskeletal pain in community-dwelling older persons. Journal of the American Geriatrics Society, 51(8):1092-1098.
Turk, D. C. and E. S. Monarch (2002). Biopsychosocial perspective on chronic pain. In: D. C. Turk & R. J. Gatchel (Ed.), Psychological approaches to pain management: A practitioner’s handbook (2nd ed., pp. 3–30). New York: Guilford Press.
Verbeek, J., M. J. Sengers, L. Riemens, J. Haafkens. (2004). Patient expectations of treatment for back pain: a systematic review of qualitative and quantitative studies. Spine, 29(20), 2309-2318.
Watson, T. (2016). Soft tissue repair and healing review. [online]: http://www.electrotherapy.org/modality/soft-tissue-repair-and-healing-review
Wernicke, S., J. Huberts, P. Wippert (2015). The pain of being misunderstood: invalidation of pain complaints in chronic low back pain patients. Journal of Health Psychology, 1–13.